What does X-Therma deal with?
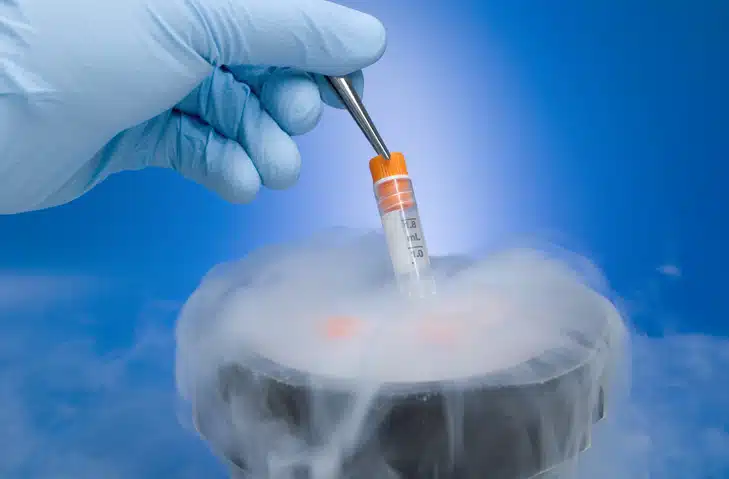
“X-Therma,” Francesca explains, “is developing a cutting-edge technology in the field of cryopreservation, inspired by the antifreeze proteins found in some Arctic species, and aims to revolutionize organ, tissue, and cell preservation. The company attempts to overcome a major challenge in both transplantation and regenerative medicine, by extending the shelf life of these organs, tissues, and cells, while at the same time maintaining their functionality. The flagship solution, which is called XT-ViVo®, uses biomimetic peptides that prevent the formation of ice crystals, therefore, protect tissues and ensure safe storage even at subzero temperatures. This technology has the potential to significantly improve transplant outcomes, while also reducing waiting lists and consequently saving more lives.”
“X-Therma,” she continues, “has a product pipeline with a clear and also ambitious development roadmap. It is currently working to finalize preclinical studies on the XT-ViVo® and X-TimeSeal® products. The goal is to obtain FDA approval to begin human clinical trials. This technology has already obtained breakthrough device status from the FDA, a crucial milestone because it accelerates the company’s regulatory pathway. The main target is hospitals, as these products are used during transplantation to enable the organ to be transported to another hospital. In parallel, X-Therma also has another product, XT-Thrive®, which has already obtained GMP certification making it ready to be marketed. This product is for cryopreservation, in cell preservation, e.g., for gene therapies, biobanking, tissue cultivation, and is already approved for the de facto unregulated market. The target market of the latter product includes CROs and companies involved in cell therapy, which need to preserve cells and tissues.”
When was X-Therma born and how is the team composed?
“X-Therma was founded in 2017,” Francesca continues, “and it is headquartered in Hercules, San Francisco Bay, California, but also has an operational office in Innsbruck, Austria. The company is already well structured, with a team of experts with multidisciplinary skills. The CEO and Co-founder is Xiaoxi Wei, a leading figure in biotechnology, while Dr. Gerald Brandacher, a world authority in organ transplantation, is the project’s scientific advisor. In addition, the team consists of 30 full-time employees with a combination of scientific, business and technological expertise. They are supported by a high-level board and various advisors, both scientifically and business-wise. In total, 30 people are operating in Hercules, while in Innsbruck the team is smaller, consisting of 5 or 6 people.”
Which are the main activities carried out in the two sites?
“In Innsbruck the team consist entirely of technicians, who work on the development of X-TimeSeal®, a device used for organ transport. This development team has reached the design freeze of the device and is currently working on getting it certified. The San Francisco office is the headquarters for all of the XT-ViVo® production development. In addition to the research and development team, they have also begun to structure a sales team, for the launch of their first product, XT-Thrive®.”
The first transatlantic kidney transport represents a revolution in this field.
“Certainly,” Francesca confirms. The kidneys were transported from Baltimore, USA, to Innsbruck, Austria, using the XT-ViVo® solution and the X-TimeSeal® device. The organs were stored for up to 72 hours at temperatures below 0°C, maintaining all its functionality. This shows that it is possible to significantly extend organ preservation time, overcoming current logistical limitations, and X-Therma thus opens up the possibility of increasing the number of successful transplants and improving the quality of preservation, and will consequently largely reduce waiting lists.”
How will the capital raised so far be used?
“The latest round was 22.5 million, bringing the total fundraising, through the various rounds, to about 50 million. The round will help cover activities and studies to achieve FDA approval for products still in the certification stage. In parallel, it will be allocated to the development of a sales network for the commercialization of XT-Thrive®.”
What will the role of LIFTT’s support be?
“LIFTT’s support will be multi-pronged,” concludes the PM. In late September, I visited X-Therma’s headquarters in the United States to understand their strategy and next steps, but more importantly, to discuss how LIFTT can support the company’s growth. Our support will focus on activating potential Italian and European partners and customers. We can facilitate access to CROs (Contract Research Organizations) and companies engaged in the field of cell therapies, thus contributing to the growth of sales and expansion of their network outside the United States. In addition, we will work together with them to develop and implement a targeted growth strategy that will help them consolidate their market position and strengthen their corporate structure and management in the coming years, with the goal of an exit in the short to medium term.